The spatial biology revolution
A new revolution in cancer research
There is a new revolution in cancer research – a spatial biology revolution that is destined to change how scientists capture information about tumours, and may lead to new ways to diagnose and treat cancer. The Cancer Grand Challenges IMAXT and Rosetta teams, tackling the 3D tumour mapping challenge, have exploited new and existing technologies to propel spatial biology research even further to the forefront of cancer science.
In 2015, Cancer Grand Challenges set the 3D tumour mapping challenge, challenging the scientific community to develop methods to map the complex interactions among heterogeneous tumour cells, immune cells, tumour cell clones and components of the tumour microenvironment.
At the time the challenge was set, single-cell RNA and DNA sequencing had just taken the field of biology by storm. It was unclear, however, whether single-cell data alone would actually lead to better prognosis and diagnosis of cancer, or provide actionable clinical data.
“If you look at where the field is going,” says IMAXT team lead Greg Hannon of the Cancer Research UK Cambridge Institute, “it’s spatial multi-omics. It will be making multiple types of measurements in a spatial manner – things like clonal distribution of cancer cells, the transcriptome and the proteome – at the same time. That will all be integrated into a larger picture.”
IMAXT and Rosetta, led by Josephine Bunch of the National Physical Laboratory, UK, took the next step of developing techniques to reveal 3D cellular and molecular maps of the mutational and structural heterogeneity in DNA, patterns of conventional and nonconventional RNA transcription and protein expression, and the biochemical signatures of cells and cell types in a tumour, while simultaneously capturing the complexity of the intra-tumour architecture.
“From a technical perspective, we have developed or matured some of the most promising spatial measurement techniques in the field,” explains Greg. “These will have lasting impact.”
Spatial technology that drives cancer research
Early on, Greg and the IMAXT team realised that no single technology would ever achieve the depth of profiling required to take on some of the toughest questions in cancer biology. The team emphasised the development of tools to integrate data from multiple technologies, enabling single-cell and spatial profiling of tumours with sufficient throughput to allow for large-scale studies. This resulted in the establishment of a world-class data processing centre in Cambridge, built out of a successful collaboration with the Institute of Astronomy.
Another critical aspect of the project was finding ways to visualise the enormous amount of data collected for each tumour and make it accessible to scientists everywhere in the world. A core component of the IMAXT programme is Project Theia, the world’s first virtual reality cancer research laboratory. Theia lets users step inside intricate 3D tumour maps and explore the tumour landscape in unparalleled detail.
By combining existing technologies with new approaches, IMAXT has developed entirely new ways to study cancer, that are changing how cancers are classified, treated and managed, and giving more people a better chance of surviving their disease.
“I have been lucky enough to witness the potential benefits of these new technologies, which enable greater examination of a cancer and its individual cell structure in either two or three dimensions,” says Elaine Chapman, IMAXT patient advocate, UK, who has been living with stage 4 cancer for 17 years. “This opens up a field in which researchers and clinical teams can jointly gain a far greater understanding of cancer, which, in time, will lead to earlier diagnosis and a wave of more targeted treatments.”
Some of the spatial biology technologies developed, or optimised for use in tumour samples, by IMAXT include:
- MERFISH (Mutiplexed Error-Robust Fluorescence In-Situ Hybridisation)
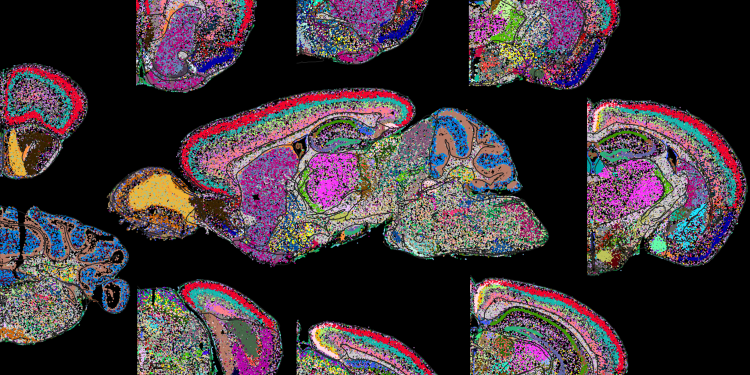
An imaging method capable of simultaneously measuring the copy number and spatial distribution of hundreds to thousands of RNA species in single cells. “With MERFISH,” says IMAXT co-investigator Xiaowei Zhuang of Harvard University, US, “we can directly image, in situ, the gene expression profile of individual cells for cell type identification and discovery. A significant advantage of MERFISH is its ability to recover functionally critical spatial information.” In Cell, Xiaowei and her colleagues described an additional MERFISH modality, epigenomic MERFISH, that enables spatially resolved single-cell epigenomic profiling. Using epigenomic MERFISH, Xiaowei generated a high-resolution atlas of hundreds of active promoters and enhancers in embryonic and adult mouse brains.
- Multiplexed immunofluorescence (mpIF)
An imaging technique that enables researchers to identify the types of immune cells in a tumour and its microenvironment. Using mpIF combined with whole-genome and single-cell sequencing, IMAXT co-investigator Sohrab Shah of Memorial Sloan Kettering Cancer Center, US, and Ignacio Vázquez-Garcia, a postdoctoral fellow in Sohrab’s laboratory, discovered a mechanism of immune evasion in distinct mutational subtypes of high-grade serous ovarian cancer tumours. In their work they show that compositional, topographic and functional differences exist among mutational subtypes. They also discovered site-specific properties of the primary tumours and distal peritoneal metastases. The findings, published in Nature, highlight the urgent need for early detection and personalised treatment approaches for ovarian tumours.
“These technologies provide an extraordinary view of the spatial dissemination and interactions of cancer cells in the context of the surrounding tissue microenvironment,” says Ignacio, lead author of the study. The Joyce group also developed a novel multiplexed immunofluorescence technique named HiFI (Hyperplexed Immunofluorescence), which enable the detection of even more markers, which is currently pending publication.
- 3D imaging mass cytometry (3D IMC)
Used for multiplexed 3D tissue analysis at single-cell resolution. Using 3D IMC, IMAXT co-investigator Bernd Bodenmiller of ETH Zurich, Switzerland, revealed cellular and microenvironmental heterogeneity and cell-level tissue organisation not detectable in 2D. The study, published in Nature Cancer, suggests that 3D IMC may be a powerful tool for studying phenomena, such as tumour cell invasion, that occur in 3D space. The detailed models generated with 3D IMC facilitate comprehensive, single-cell-resolution analysis of cellular microenvironments and tissue architecture.
- WILD-seq (Wholistic Interrogation of Lineage Dynamics by sequencing)
A novel strategy for lineage tracing (a way of following the evolutionary history of cell clones in tumours) coupled with single-cell transcriptomics to enable insights into tumour heterogeneity and response to treatment. In a study published in eLife, the IMAXT team used WILD-seq to identify a major mechanism of chemotherapy resistance in mouse models of triple-negative breast cancer. Their studies revealed asparagine bioavailability as a druggable vulnerability of taxane-resistant clonal lineages.
IMAXT’s efforts in developing these technologies and the expertise to study the complexity of tumours at the 3D level have enabled the team to begin to address important and fundamental questions in cancer biology that otherwise would not have been possible.
Mapping metabolites in the microenvironment
Rosetta adds a metabolic view to spatial biology. The team is using mass spectrometry imaging (MSI) to map the spatial distribution of cellular metabolites in the tumour microenvironment in exquisite detail. The information uncovered through MSI provides a deeper understanding of tumour behaviour, progression and response to therapy by mapping metabolite distribution in relation to individual cells’ genetics and the overarching tumour architecture.
“Mass spectrometry imaging existed before the Cancer Grand Challenges programme,” says Josephine, “but no project had ever really allowed it to be deployed at such scale, bringing as many different modalities together. Throughout the programme, improvements in sample handling, introduction of new modalities and novel data handling and protocols to integrate these measurements have culminated in a very powerful pipeline.”
Rosetta’s pipeline has begun to demonstrate the clinical application of metabolic profiling and how this technique could be used for patient stratification, monitoring responses to therapy and drug discovery.
Utilising MSI technology, Rosetta co-investigator Mariia Yuneva of The Francis Crick Institute, UK, and former team member Peter Kreuzaler of the University of Cologne, Germany, identified pantothenic acid (vitamin B5) as a metabolite biomarker associated with high expression of MYC in breast cancer. Use of the entire Rosetta MSI pipeline, from low to high spatial resolution, enabled them to see where atoms from specific molecules, such as pantothenic acid, are localised within tumour tissue. In their recent article published in Nature Metabolism, they showed that MYC increased levels of vitamin B5 in tumours by upregulating the expression of the multivitamin transporter SLC5A6.
When the researchers put mice on a pantothenic acid-deficient diet, they found that both mouse tumours and patient-derived-xenograft models of breast cancer grew more slowly. This finding suggests that decreasing vitamin B5 availability to the tumour may be advantageous. “All the growth advantage that cells gain by upregulating MYC was lost by taking away one single vitamin,” says Peter. This finding could open up new therapeutic avenues for MYC-driven cancers.
In another study published in Nature Metabolism, Rosetta researchers showed that metabolic profiling using a variety of mass spectrometry techniques can be used to stratify tissues according to their underlying mutations in colorectal cancer, and that such techniques can identify new potential targets for cancer treatment.
Johan Vande Voorde, former team member of Rosetta co-investigator Owen Sansom of the Cancer Research UK Scotland Institute (formerly the Beatson Institute), identified an enzyme, called adenosylhomocysteinase (AHCY), that is upregulated in colorectal cancer compared to with normal intestine, and may be a potential treatment target, according to preclinical findings.
“Our results provide preclinical and clinical evidence that we can use the metabolic profile of intestine samples to understand what the drivers are within a tissue,” says Owen.
The researchers plan to validate their findings in a larger patient study, and aim to test whether targeting AHCY in particular subtypes of colorectal cancer might provide patient benefit.
Johan notes that their studies will explore AHCY as a potential cancer target in more complex models and examine other processes, such as metastasis, to determine how broadly this intervention could be applied as a new target.
The power of the Rosetta pipeline and its potential for clinical impact has been demonstrated through its application to AstraZeneca’s drug development pipeline.
“The Rosetta platform is an integral part of the AstraZeneca spatial-omics workflow and suite of capabilities, which help us to answer important scientific questions,” says Simon Barry, Rosetta co-investigator and Executive Director at AstraZeneca, UK. “We apply the technology across a range of studies – from preclinical to biomarker and mechanistic studies – to gain insight and predict clinical impact.”
“Rosetta has exemplified the value of collaboration in combining a broad set of technologies and approaches,” adds Richard Goodwin, Senior Director at AstraZeneca and Rosetta co-investigator. “The results of these efforts are truly changing the field and have made it easier to integrate the transformational technologies into clinical decision making, with potential for patient benefit.”
An unprecedented view of tumour complexity
As the spatial biology revolution in cancer evolves, IMAXT and Rosetta are continuing to demonstrate the power of spatial profiling for cancer research. “Together, these teams have given us a ‘Google Earth'-like tool to study the anatomy of cancers at an extraordinary level of detail,” says René Bernards of the Netherlands Cancer Institute and member of the Cancer Grand Challenges Scientific Committee. “These technologies hold great promise to better understand the interactions between tumours and their microenvironments.”
Since the 3D Tumour Mapping challenge was set, a new era of cancer biology has emerged, characterised by the widespread adoption of singlecell technologies and the gradual emergence and affirmation of spatial-omics technologies. The platforms developed by IMAXT and Rosetta have provided an unprecedented view of tumour complexity, allowing for the identification of new targets for drug development, advancing understanding of why some tumours become resistant to treatment and driving insights that could improve outcomes for patients worldwide.
This article, written by Scott Edwards, was originally included in our annual progress magazine, Discover: a year of scientific creativity.
Image: MERFISH image of whole mouse brain cell atlas. Credit: Zhuang lab.
Related Content
A holistic approach to cancer research: bringing future research leaders together with patient advocates
Q&A with patient advocates and future leaders.
The power of combining mass spectrometry techniques to interrogate the cancer metabolome
Q&A with Dr Johan Vande Voorde of team Rosetta.



