A breakthrough in digital breast pathology: the journey to AIDmap
New publication from team PRECISION.
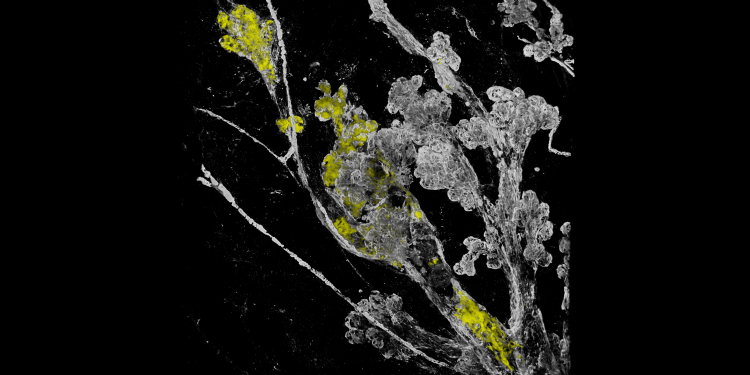
Today in Nature, Team PRECISION published its findings, Mechanisms that clear mutations drive field cancerization in mammary tissue. Led by Colinda Scheele and Jacco van Rheenen of Cancer Grand Challenges team PRECISION, together with Ben Simons, the work shows multiple layers of protection exist to stop mutated clones from spreading within the breast, identifying the molecular mechanisms behind why having fewer menstrual cycles is associated with decreased breast cancer risk. Here we highlight the impact of their findings, discuss the game-changing techniques that allowed the visualisation of the very early stages of tumour formation, as well as the journey of Colinda from PhD student through to group leader over the course of this work.
It’s been a long journey. Colinda Scheele originally began this project as a PhD student in Jacco van Rheenen’s lab at the Netherlands Cancer Institute. Originally a Master’s student in a neighbouring lab, Jacco had invited Colinda to discuss her joining his lab for her PhD, after hearing about her from a post doc on his cycle into the institute. But whenever she went to his office he was busy. After five attempts over multiple days, they managed to have their meeting. Jacco recalls, ‘It was immediately clear that Colinda was phenomenal.’
Colinda’s persistence paid off; Jacco offered her a position. And this persistence has carried her through ever since.
Colinda recalls, ‘I joined Jacco's lab because he's known for being an expert in intravital microscopy, really being able to visualise cells in their native environment in real time. I first studied the behaviour of healthy stem cells in the breast and then of course a very logical next step was to see what happens if the cells have a mutation. And that's also where PRECISION came in: asking what happens with very early mutant cells actually before they make a tumour mass.’ The PRECISION team was funded in 2017 by Cancer Research UK and the Dutch Cancer Society (KWF) through Cancer Grand Challenges to tackle the lethal versus non-lethal cancers challenge, focusing on the breast and ductal carcinoma in situ (DCIS), to determine when we call something cancer and, critically, when we need to do something about it.
Findings emerging at the time, including from team Mutographs, had shown that many ‘normal’ cells from healthy individuals carry oncogene driver mutations. The initial question for this work was, as Jacco puts it, ‘How is that possible?!’ Why do these mutations not always lead to cancer? What are the mechanisms at play that protect the breast from the accumulation or spread of cells carrying driver mutations?
To get at this question Colinda first painstakingly developed and optimised techniques to allow the team to induce mutations stochastically throughout the breast in mice, in just a few cells, and then follow their behaviour using lineage tracing. In the past, people had generally mutated entire tissues or animals in one go. Colinda’s approach would more accurately reflect the natural situation, where cells acquire mutations at random over time, whilst being surrounded by otherwise normal tissue. But Colinda also then needed to find the mutated cells. As Jacco reflects, ‘If you induce mutations in a whole tissue, you just take a tissue section and you can study whatever you like. But now in our case we have the whole organ with only a few mutant cells, and we have no idea where these cells are. So what Colinda had to do is also develop a technique where she takes the whole tissue in 3D and now scans it all to find them. And that's also something unique.’
Colinda was setting up for a marathon, not a sprint. She reflects that Cancer Grand Challenges ‘make[s] it possible to think big and as a PhD student back then you also really felt that it was possible to tackle the research question in in a way that you could not tackle it before with smaller grants and smaller budgets.’
Using these game-changing techniques the team induced the sporadic loss of the tumour suppressors brca1 or p53, and followed the mutated cells. The team did find that some of these mutated cells formed tumours, but these were the minority, discovering multiple layers of protection exist to stop mutated clones from spreading. This was the same for clones carrying both oncogenic and neutral mutations.
As the tissue is constantly turning over, the first layer of protection is provided due to the fact that the majority of cells are only short-lived. So if a mutation is acquired within these short-lived cells, they simply disappear after a few rounds of proliferation. If a mutation is acquired in one of the longer-lived cells, those with stem cell properties, these cells are retained and the mutation can spread to their progeny.
Then the second layer comes into play. Tissue remodelling driven by the oestrous cycle, the equivalent of the menstrual cycle in mice. As Jacco describes it, ‘the tissue is remodelled and it looks like a tree where during the spring you get leaves being formed, that then fall off in the autumn. And that happens during the oestrous cycle as well, the body prepares itself for becoming pregnant and if that happens, the leaves grow out into units that form the milk. But in the moment the body doesn't get pregnant, they are disappointed, so they fall off, and this is repeated, repeated, repeated.’
As this process happens along the ducts, there are cycles of amplification and depletion of stem cells. In that way most of the mutant cells disappear.
But this mechanism is in fact a double-edged sword. On the whole it protects the body, but for the few clones that do survive, it is the same mechanisms that drive their expansion, allowing them to spread over a large area, and cause a higher risk of tumourigenesis.
The team went on to show that the more frequently and the more often the mouse goes through the oestrous cycle, the higher the chance that one of these clones spreads to the extent that you get a chance of tumour formation. In fact it showed that ovariectomy reduces the risk of transformation in the mice.
It was known that having fewer menstrual cycles was associated with decreased breast cancer risk, so women who have longer cycles, who enter the menopause earlier, or start their menstrual cycles later, are thought to have a decreased risk 1-4. However, the molecular mechanisms underlying this phenomenon were, until know, unknown.
The team’s findings really highlight the need for thinking of cancer as systemic disease. As Jacco puts it, ‘The whole body is important for the risk and not only the molecular change in the DNA.’
The third layer of protection is provided by the geometry of the ducts, that restricts the expansion. As Jacco describes this final layer, ‘There is even a constrain in the morphology of the tissue that stops the mutant cell from spreading too much.’
Upon seeing these areas of clonal expansion, Colinda reflects, ‘I remember we saw these large fields in the mouse mammary glands and we discussed it with Jelle Wesseling, a human breast pathologist, and PRECISION team lead. And he said, “Oh, this looks like the sick lobe theory – it has been around for years, but we were never really able to prove this.”’
Field cancerisation, also known as the sick lobe theory in breast, is where cells containing oncogenic mutations first spread over vast ductal areas of the breast and are thought to sensitise the epithelium to future transformation. So you first need a field with mutant cells to then transform into a tumour.
Jacco recalls:
"When Colinda was doing her first experiments, we had no idea about this early phase of tumourigenesis. And then with PRECISION, we got in the team people who study this in mice – in Jos Jonkers’ lab – and in the human setting – including Jelle Wesseling and his team, but also many others – who could really help to interpret our data. We had many discussions that were instrumental, also in how to make this relevant to the human setting.’
Moving to human samples, through PRECISION, in work also led by Colinda, the team found further support for the sick lobe theory. To enable this work (published in The Journal of Pathology), Colinda’s lab again first developed methods to allow 3D reconstructions of the human breast from individuals with DCIS, and, critically, combine this with spatially resolved DNA copy number aberration (CNA) sequencing. This allowed the team to map the extent and dynamics of the spread of mutations, and provide further evidence for the clinical significance of field cancerisation.
Colinda reflects, ‘It was super important to have clinicians, but also basic scientists together. PRECISION really allowed that. By combining our efforts it really brought these stories to the next level.’
Jacco adds, ‘You need the input from so many different people, so much different expertise.’ Ben Simons was another person who was critical for their Nature paper, making a biophysical model to fully explain all the phenotypes the team had observed in vivo.
Almost nine years since she first began the project, Colinda reflects, ‘It takes a very long time to look at a few cells and see what they do 1.5 years later. So in the end, the project just carried on and my career also continued. In 2020, I finished my PhD, I finished in Jacco’s lab. But the project was still ongoing, and so I continued in my own lab, at VIB in Belgium, now as a collaboration. It was really a huge collaborative effort with multiple people involved, also from my lab and from Jacco’s lab.’
As team PRECISION’s Cancer Grand Challenges funding comes to an end, Colinda’s lab continues to work on the mammary gland and these early transformation events, as well as looking into other remodelling events, such as pregnancy. They are also starting to explore how metastatic cells reprogramme their environment at distant sites. As Colinda puts it, ‘There are so many unanswered questions, but with the models we developed we now have great tools in our hands to answer them.’
Reflecting on Colinda’s success, Jacco comments, ‘Colinda is already outperforming me, my legacy is already there. I'm extremely proud. It’s extremely important, the next generation is what we need.’
-----------------
Article written by Rebecca Eccles with thanks to Colinda Scheele and Jacco van Rheenen.
Hero image: Fields of mutant cells (in yellow) are sensitive to transformation, starting with subtle changes and deformation in the mammary gland structure (in white). Credit: Colinda Scheele
Learn more about team PRECISION’s work.
New publication from team PRECISION.
Bringing us closer to distinguishing harmless from hazardous DCIS.