A breakthrough in digital breast pathology: the journey to AIDmap
New publication from team PRECISION.
In 2015, Cancer Grand Challenges set the lethal vs. non-lethal cancers challenge with the vision to develop a thorough understanding of features that can distinguish a non-lethal growth from a potentially lethal malignant growth, so that methods could be developed to specifically detect cancers that require intervention. The PRECISION team has been tackling this challenge in ductal carcinoma in situ (DCIS), a potential precursor of invasive breast cancer. By taking a deep dive into DCIS biology, the team has transformed the DCIS field, bringing us closer to distinguishing harmless from hazardous DCIS.
Advances in screening methods mean that we can now detect some cancers, such as breast and prostate cancer, at an early stage. Although this capability is highly beneficial in the cancers that would develop into life-threatening malignancies, the sensitivity of screening methods has led to over-diagnosis and subsequent over-treatment in cases that wouldn’t have become dangerous.
This over-treatment is particularly true for DCIS, in which some cells in the milk ducts of the breast become abnormal but have not started to spread to surrounding breast tissue. Although DCIS is not life-threatening, and its chances of progressing to cancer are low, each year, thousands of patients with DCIS worldwide are treated as if they have invasive breast cancer, and undergo surgery, hormonal and radiotherapy.
“Over the years, it has become clear that up to 4 out of 5 DCIS lesions will never progress to invasive breast cancer if left untreated,” says PRECISION team lead Jelle Wesseling of the Netherlands Cancer Institute (NKI). “That implies that the majority of women with a DCIS diagnosis undergo invasive treatment without any benefit.”
Therefore, the PRECISION team has taken a comprehensive approach to develop the knowledge and tools that could help determine which DCIS cases need treatment and prevent the consequences of over-treatment.
In the clinic, DCIS size and margin status are two factors often used to stratify the risk of DCIS lesions and determine the course of treatment. However, in a recent study published in the British Medical Journal, PRECISION researchers found only a weak association between these clinical factors and the risk of subsequent ipsilateral invasive breast cancer. The team combined data from more than 47,000 women with DCIS from the Netherlands, UK and US, who had received either breast-conserving surgery or mastectomy, often followed by radiotherapy or hormone treatment, or both.
The goal of PRECISION is to de-escalate DCIS treatment, however, the associations reported in our study are not large enough to drive clinical decisions regarding which patients should be treated and which patients could safely not be, explains Marjanka Schmidt of the NKI, PRECISION co-investigator and senior author of the study.
Part of PRECISION’s goal has been to shift perceptions of a diagnosis of DCIS away from early breast cancer. In the same study, which was the largest of its kind to explore the value of prognostic risk factors after DCIS, the team reported that the 10-year cumulative incidence of ipsilateral invasive breast cancer was just 3.2%.
“Women need much more information about their individual risks from DCIS before they make treatment decisions,” says PRECISION patient advocate Hilary Stobart, UK. “PRECISION is working hard to resolve this dilemma by working together to find a combination of biomarkers, making very important steps towards achieving this goal so that each woman will soon be able to know her own individual risk.”
To understand the biochemical and genetic pathways that drive cancer, researchers rely on accurate disease models. However, no sufficient models of DCIS had been available to study disease progression until recently.
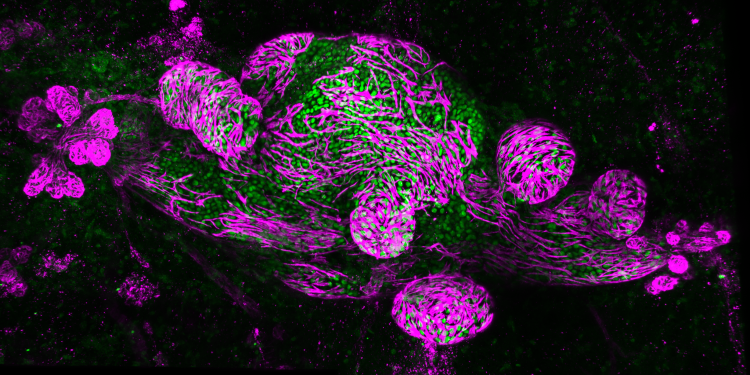
The Mouse INtraDuctal (MIND) model is the first in vivo model for studying the natural evolution of DCIS. It was developed by PRECISION co-investigator Jos Jonkers and his team at NKI in collaboration with other PRECISION co-investigators, including Fariba Behbod of the University of Kansas Medical Center, US. In this model, patient-derived DCIS epithelial cells are injected into mice to mimic patient histology and biomarker expression.
The PRECISION team has developed a living biobank of 115 patient-derived MIND models and used them to identify risk factors associated with the progression of DCIS to invasive breast cancer. Using multiple omics methods, the researchers compared two groups to characterise the differences between DCIS cases that progressed to invasive cancer and those that did not.
“We saw two distinct growth patterns – replacement growth, which didn’t change the architecture of the mammary gland when the lesion grew, and expansive growth, where the lesion grew perpendicular to the ductal system and put a lot of pressure on the ducts,” explains Jos, senior author of the study. “The latter growth pattern may eventually lead to the breakage of the duct and correlate with invasive progression.”
The study, published in Cancer Cell, provides critical information about which markers might potentially predict invasive progression in women with DCIS. Importantly, notes Jos, the biobank includes 19 distributable DCIS MIND models, accessible through CancerTools.org, which have been made available to researchers worldwide to further investigate DCIS molecular subtypes.
“As we and other research groups have shown, most DCIS cases will not become dangerous,” says PRECISION’s Stefan Hutten, postdoctoral researcher at NKI and first author of the Cancer Cell article. “I hope, in the future, we will be able to better assess prognosis in DCIS patients and divide them into three groups: a low-risk group, which receives active surveillance; a high-risk group, which should receive the current standard of care; and a medium-risk group, where the physician makes an informed decision together with the patient about the course of action.”
In another effort to avoid unnecessary treatment of DCIS, Elinor Sawyer of King’s College London, UK and Jelle, together with colleagues from PRECISION have been working to develop a prognostic classifier that can identify women with DCIS who are unlikely to develop invasive breast cancer. Using RNA sequencing, they have identified more than 100 genes whose expression varies depending on the risk of breast cancer recurrence after DCIS.
According to the expression of these genes, the predictor divides patients into three categories: low risk, intermediate risk and high risk of disease progression. “Rather than having an operation to remove their DCIS,” says Elinor, “women in the low-risk group could follow a watch-and-wait policy with annual mammograms because they are very unlikely to develop invasive breast cancer in the future.”
The predictor may also help to identify women at low or intermediate risk of DCIS progression who do opt for surgery but derive little benefit from additional radiotherapy.
The PRECISION predictor is being validated using samples from COMET (Comparison of Operative versus Monitoring and Endocrine Therapy), a US-based randomised clinical trial for people with low-risk DCIS. “It’s only when we get these results back that we will be able to say whether it’s safe for women in the low-risk group not to have surgery, but to undergo regular surveillance,” adds Elinor.
PRECISION’s approach is complementary to a tool being developed by one of its co-investigators, Shelley Hwang of Duke University, US, as part of her involvement in the National Cancer Institute’s Human Tumor Atlas Network (HTAN). In work related to HTAN’s Breast Pre-Cancer Atlas, Shelley and colleagues identified 812 genes associated with ipsilateral DCIS recurrence within five years of treatment, as well as stromal expression patterns and immune cell compositions. These markers successfully predicted the risk of both overall recurrence and invasive progression.
“The really exciting part of all of this is that we feel the markers may have promise in terms of predicting which patients may not need to have surgery at all, because their risk of developing cancer is so low,” says Shelley. “These types of biomarkers would address an important clinical need, allowing us to reserve surgery only for those patients who would benefit substantially from it.”
The Cancer Grand Challenges’ team-science approach is helping PRECISION meet the challenge of distinguishing DCIS lesions that require treatment from those that don’t. For example, with Serena Nik-Zainal of Cambridge University, UK, PRECISION is working with the eDyNAmiC team to determine whether extrachromosomal DNAs (ecDNAs) are present in samples from patients with DCIS that ultimately progresses to invasive breast cancer.
With Serena Nik-Zainal of Cambridge University, UK, PRECISION is working with the eDyNAmiC team to determine whether extrachromosomal DNAs (ecDNAs) are present in samples from patients with DCIS that ultimately progresses to invasive breast cancer. Serena, who is a co-investigator in both teams, has applied tools developed by the eDyNAmiC team to show that a high proportion of the DCIS samples PRECISION has studied – about 50% – contain ecDNA. She is now validating the ecDNAs PRECISION has identified and applying eDyNAmiC’s newest algorithms to understand the structure, origin and function of ecDNA in DCIS.
“This cross-team collaboration is critical,” says Serena. “From our first meeting, it was clear that the expertise from the two teams was critical for us to understand our observations about ecDNA going forwards.”
“DCIS overtreatment is such a big problem that others within the Cancer Grand Challenges community are tackling it, too,” says Jelle. “One team doesn’t have all the answers, but collaborations within the Cancer Grand Challenges community and beyond are leading to important progress and further research in this area, and sparing thousands of women with DCIS from needless treatment.”
PRECISION’s comprehensive approach to tackling the Lethal versus Non-Lethal Cancers challenge has transformed the DCIS field. The knowledge, models and resources developed by the team are paving the way for the future of the field, helping to reduce the burden of DCIS overtreatment and improve quality of life for thousands of people across the globe.
This article, written by Scott Edwards, was originally included in our annual progress magazine, Discover: a year of scientific creativity.
Image: Whole-mount image of DCIS MIND model, with DCIS cells in green and myoepithelial cells in magenta, which represent the ductal tree. Credit: Stefan Hutten and Colinda Scheele.
New publication from team PRECISION.
Highlighting the impact of new findings from PRECISION, discussing the game-changing techniques that allowed the visualisation of the very early stages of tumour formation, and…